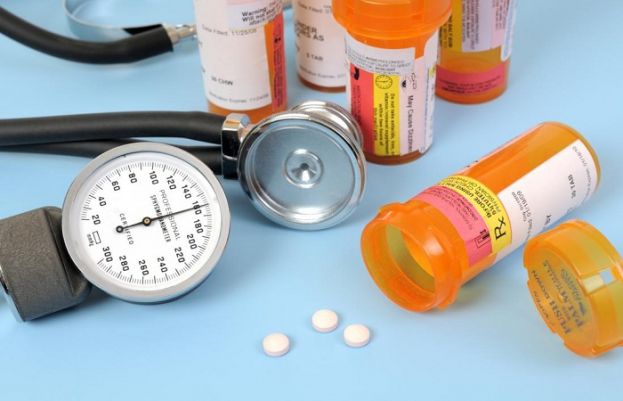
Numerous blood pressure drugs are now involved in the recall that began in late July over a suspected cancer-causing compound.
Another company has announced a recall of a blood pressure medication due to contamination concerns.
Macleods Pharmaceuticals Limited has informed the Food and Drug Administration (FDA) that it is voluntarily recalling one lot of Losartan Potassium/Hydrochlorothiazide 100mg/25mg combination tablets.
Company officials said they detected trace amounts in the products of an impurity called N-nitrosodiethylamine (NDEA), a pharmaceutical ingredient the FDA has classified as a "probable human carcinogen."
The Losartan tablets are used to treat hypertension and hypertensive patients with left ventricular hypertrophy.
Macleods officials said they have had no reports of anyone with adverse effects from the recalled products.
from Health - SUCH TV https://ift.tt/2NygfD1



0 comments: